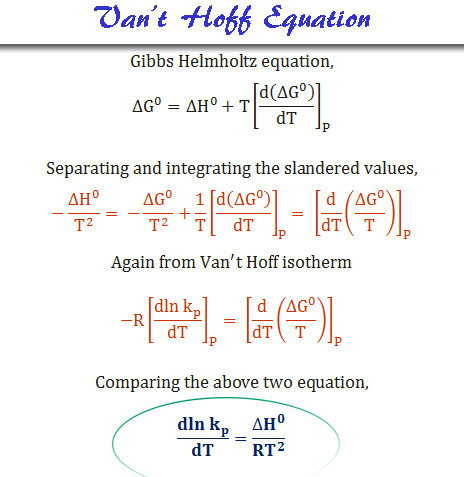
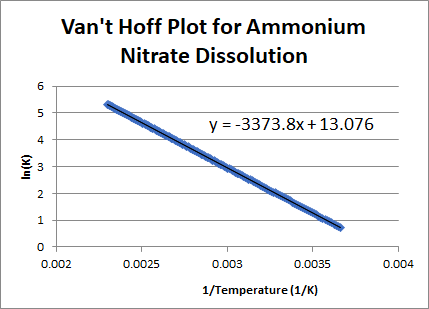
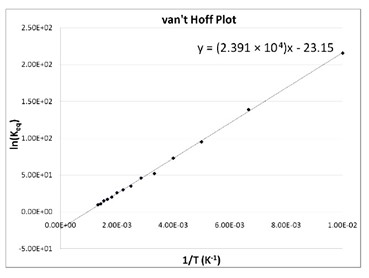
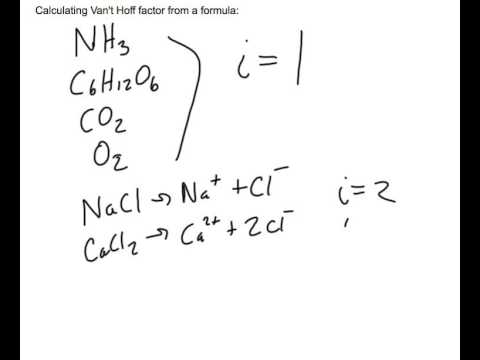
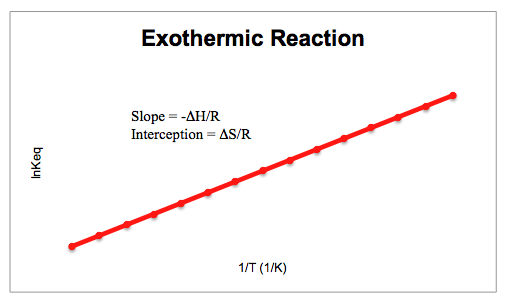
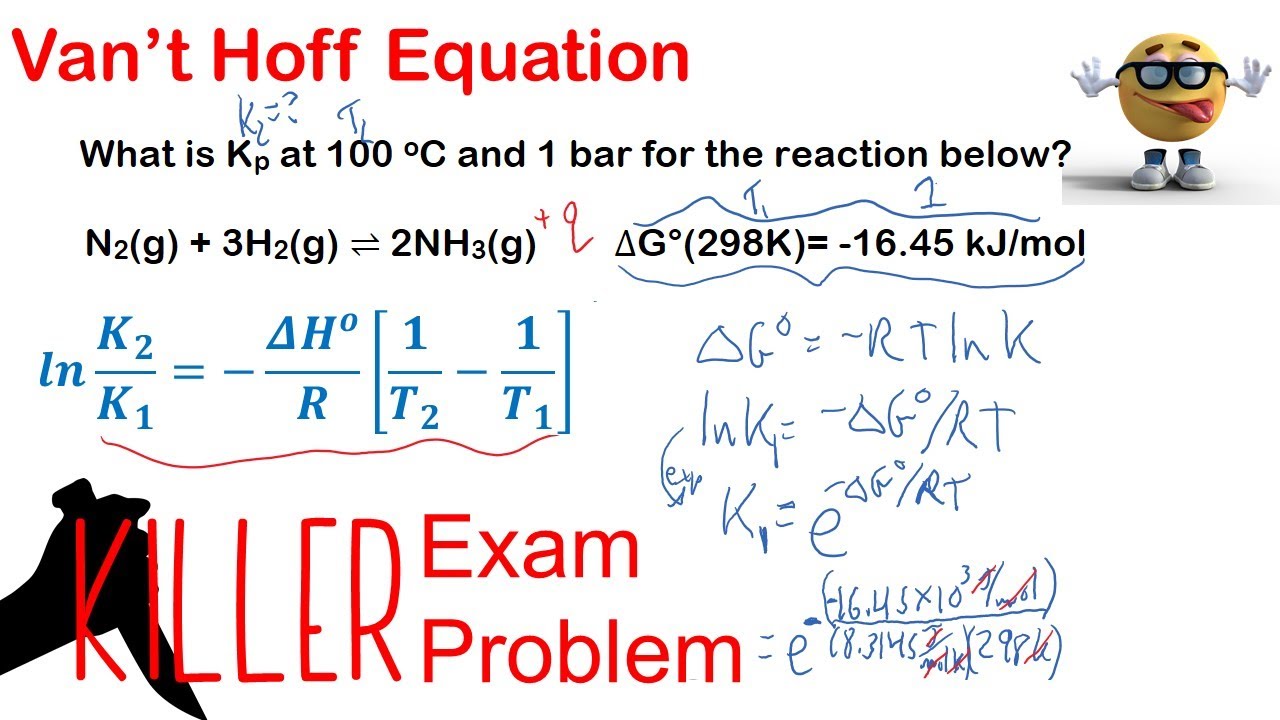
The Van't Hoff factor of 0.1 M {text{Ba}}{left( {{text{N}}{{text{O}}_3}} right)_2} solutions is 2.74. The degree of dissociation will be 91.3%74%87%100%

Jacobus Henricus Van't Hoff (1852-1911) Dutch chemist. Winner of first Nobel prize for chemistry, 1901. Engraving, 1902 Stock Photo - Alamy